Chapter 11 of Class 8 Science, "Chemical Effects of Electric Current," explores how electric current can cause chemical changes in substances. It explains concepts like electrolysis, electrodes, and the role of solutions in conducting electricity. This chapter simplifies the science behind plating metals, purifying substances, and everyday applications of electric current with practical examples and experiments.
1) Why is touching an electrical appliance with wet hands dangerous?
Answer: Wet hands can conduct electricity, which may lead to an electric shock.
2) What are good and poor conductors of electricity?
Answer: Good conductors of electricity are materials that allow electric current to pass through them, such as metals like copper and aluminum.
Poor conductors of electricity are materials that do not allow electric current to pass through them easily, such as rubber, plastic, and wood.
3) What tool was used in Class VI to test the conductivity of materials?
Answer: A tester was used to check whether a particular material allows electric current to pass through it.
4) What conclusion was drawn using the tester?
Answer: Metals like copper and aluminum conduct electricity, while materials like rubber, plastic, and wood do not.
5) Can the tester be used to test liquids for conductivity?
Answer: Yes, the tester can check if liquids allow an electric current to pass through them.
6) What precaution is mentioned regarding the use of electrical testing?
Answer: One should not experiment with the electric supply from mains, a generator, or an inverter. Only electric cells should be used for the activities suggested.
7) What should we use instead of a cell in the tester?
Answer: Replace the cell with a battery.
8) What precautions should be taken while checking the tester?
Answer: Do not join the free ends of the tester for more than a few seconds, as it may drain the battery quickly.
9) How can we test the conductivity of liquids like lemon juice or vinegar?
Answer: Collect small plastic or rubber caps of discarded bottles and clean them.
Pour one teaspoon of lemon juice or vinegar into one cap.
Dip the ends of the tester into the liquid, ensuring the ends are not more than 1 cm apart and do not touch each other.
Observe whether the bulb glows.
10) What does it indicate if the bulb glows during the test?
Answer: If the bulb glows, it means the liquid (lemon juice or vinegar) conducts electricity, completing the circuit of the tester.
11) How can we classify lemon juice or vinegar based on the test?
Answer: If the bulb glows, lemon juice or vinegar is a good conductor of electricity.
If the bulb does not glow, it is a poor conductor of electricity.
12) Why might the bulb not glow even if the liquid conducts electricity?
Answer: If the current through the circuit is too weak, the filament of the bulb does not heat up enough to glow.
13) Why does the bulb glow when an electric current passes through it?
Answer: Due to the heating effect of the current, the filament of the bulb gets heated to a high temperature, causing it to glow.
14) Why might the current in the circuit be too weak to make the bulb glow?
Answer: Some materials may conduct electricity but not as easily as metals. As a result, the circuit may be complete, but the current is too weak to heat the filament enough to make the bulb glow.
15) How can we detect a weak electric current in a circuit?
Answer: A tester that uses the magnetic effect of electric current can be used to detect weak currents. The deflection of a compass needle indicates the presence of current, even if it is small.
16) How can we modify the tester to detect weak currents?
Answer: Use an LED instead of a bulb. LEDs can glow even with a weak current.
Use a tester based on the magnetic effect of current, where a compass needle detects the flow of current.
17) What is an LED, and how should it be connected to the circuit?
Answer: An LED is a Light light-emitting diode that glows even with a weak current.
It has two leads: a longer one (connected to the positive terminal of the battery) and a shorter one (connected to the negative terminal).
18) How can we test the conductivity of liquids using the magnetic tester?
Answer: Dip the free ends of the tester into lemon juice or other liquids (e.g., tap water, vegetable oil, milk, honey).
Observe whether the compass needle deflects.
After each test, wash and dry the ends of the tester before testing the next liquid.
19) What observations should be recorded while testing liquids with the magnetic tester?
Answer: Record whether the compass needle shows deflection for each liquid tested. This indicates whether the liquid conducts electricity.
20) Why is it important to wipe the ends of the tester after testing each liquid?
Answer: To avoid mixing liquids and to ensure accurate results for each test.
21) How can we classify liquids as good or poor conductors?
Answer: Observe whether the compass needle shows deflection when the free ends of the tester are dipped into the liquid.
If the needle deflects, the liquid is a good conductor.
If it doesn’t, the liquid is a poor conductor.
22) What does the air gap between the free ends of the tester indicate?
Answer: Air is a poor conductor of electricity. However, under certain conditions, such as during lightning, air can conduct electricity.
23) Why is it better to classify materials as good conductors and poor conductors instead of conductors and insulators?
Answer: Most materials can conduct electricity under certain conditions. Classifying them as good or poor conductors acknowledges that even poor conductors may allow electricity to pass under specific conditions.
24) What is observed when distilled water is tested for conductivity?
Answer: Distilled water does not conduct electricity.
25) What happens when salt is dissolved in distilled water?
Answer: The resulting salt solution conducts electricity because the dissolved salt provides ions that allow the flow of electric current.
26) Why does tap water conduct electricity, but distilled water does not?
Answer: Tap water contains impurities such as salts and minerals that make it conductive, while distilled water is pure and lacks such impurities, making it non-conductive.
27) Why should we avoid handling electrical appliances with wet hands or on a wet floor?
Answer: Wet hands or a wet floor increase the risk of electric shock because water containing salts (like tap water) is a good conductor of electricity.
28) What substances, when dissolved in distilled water, can make it a good conductor?
Answer: Substances like acids, bases, and salts, when dissolved in distilled water, can make it a good conductor of electricity.
29) What happens when an electric current flows through a conducting solution?
Answer: When an electric current flows through a conducting solution, it produces certain effects, which can be explored further.
30) What are the electrodes in the setup?
Answer: The carbon rods with their metal caps connected to the battery act as electrodes.
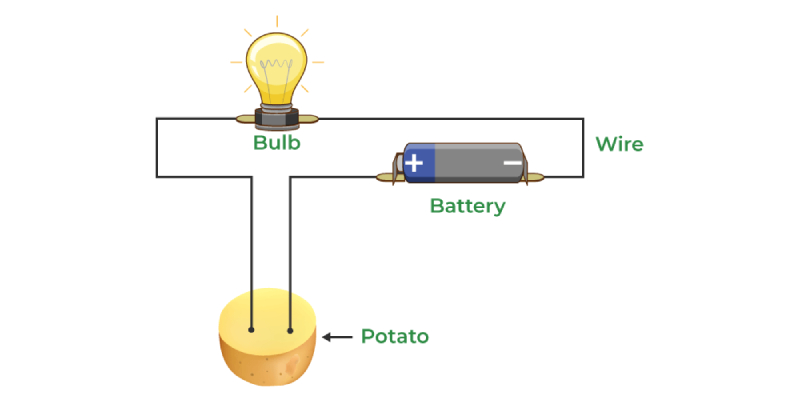
31) What chemical effect of electric current did a student observe using a potato?
Answer: The student observed that passing an electric current through a potato produced a chemical effect, which was an exciting discovery.
32) Why does the shiny coating on bicycle handlebars or gold-plated ornaments wear off?
Answer: The shiny coating wears off because it is a thin layer of metal, and with repeated use or scratching, the underlying less shiny metal is exposed.
33) What is electroplating?
Answer: Electroplating is the process of depositing a layer of one metal over another using an electric current.
34) What materials are needed for the electroplating activity?
Answer: Copper sulfate
Two copper plates (10 cm x 4 cm)
Distilled water (250 mL)
Beaker
Dilute sulphuric acid
Sandpaper
Battery
35) What happens during the electroplating process?
Answer: When current passes through the copper sulfate solution, it dissociates into copper and sulfate ions.
The free copper ions are attracted to the electrode connected to the negative terminal of the battery and get deposited on it.
Simultaneously, copper from the positive electrode dissolves into the solution, maintaining the balance of copper ions in the solution.
36) What observation is made after passing the current for 15 minutes?
Answer: A copper coating forms on the electrode connected to the negative terminal of the battery.
37) What happens when the electrodes are interchanged in the setup?
Answer: When the electrodes are interchanged, the electrode now connected to the negative terminal will receive the copper coating, while the other electrode will dissolve copper into the solution.
38) Why is copper sulfate solution mixed with dilute sulphuric acid?
Answer: Dilute sulphuric acid increases the conductivity of the copper sulfate solution.
39) How does copper sulphate dissociate in the solution during electroplating?
Answer: Copper sulfate dissociates into copper (Cu²⁺) and sulfate (SO₄²⁻) ions. The copper ions are drawn to the negative electrode, where they get deposited.
40) How is the loss of copper in the solution compensated during electroplating?
Answer: Copper from the positive electrode dissolves into the solution, maintaining the balance of copper ions.
41) What are some common applications of electroplating?
Answer: Coating metal objects with a thin layer of a different metal.
42) Why is chromium plating often used on objects like car parts and kitchen appliances?
Answer: Chromium has a shiny appearance and resists corrosion and scratches, but it is expensive.
43) How does electroplating help in the case of jewelry?
Answer: It allows for the appearance of silver or gold at a lower cost.
44) What is the purpose of tin plating on iron cans?
Answer: To prevent food from coming into contact with iron and spoiling.
45) Why is zinc plating used on iron structures like bridges and automobiles?
Answer: To protect iron from corrosion and rust.
46) What is a major environmental concern in electroplating factories?
Answer: The disposal of the used conducting solution, which is a polluting waste.